Phosphatases
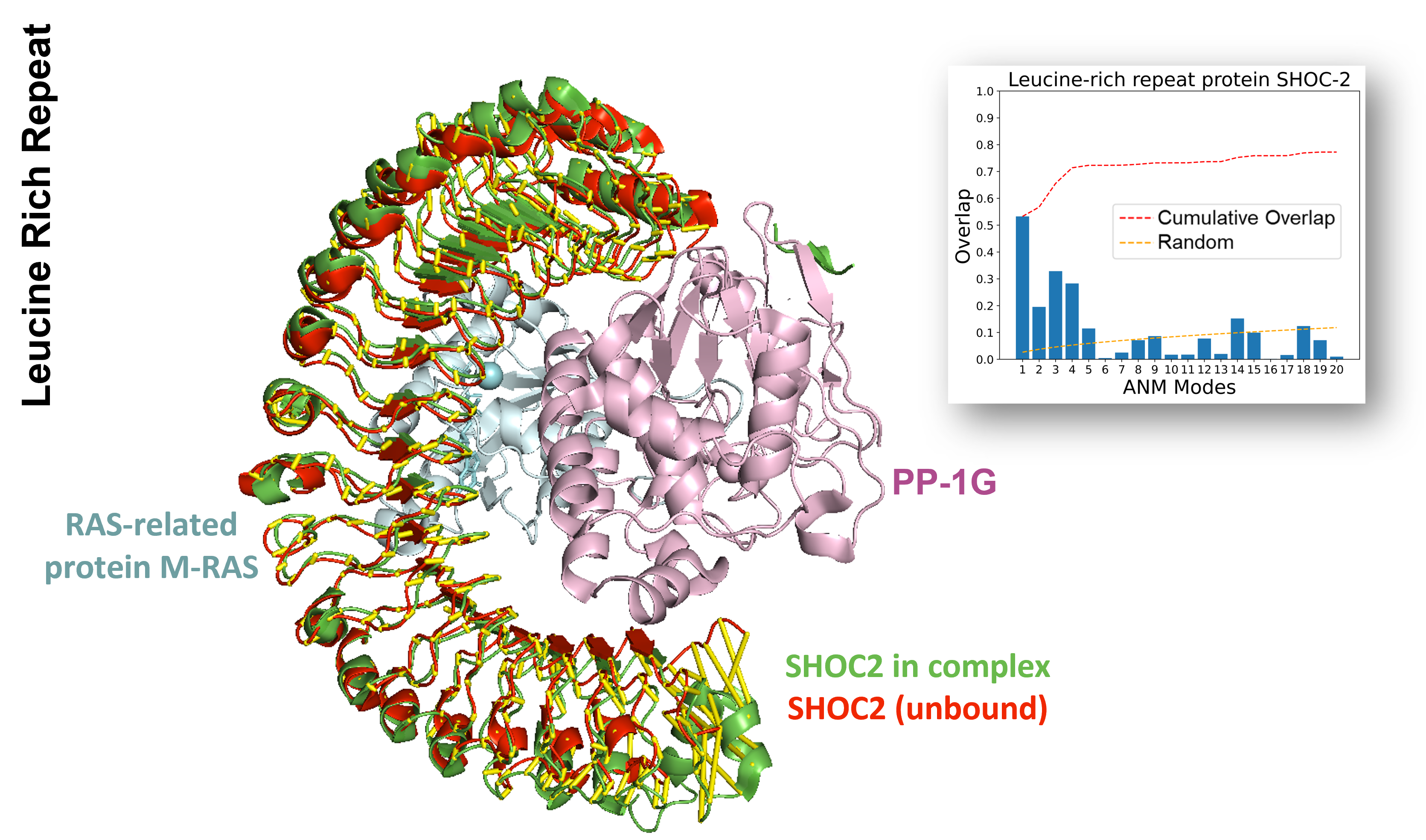
Protein phosphatase 1 catalytic subunit (PP1C)1 is a serine/threonine phosphatase that targets a wide range of proteins involved in cell cycle control, glycogen metabolism, muscle contraction, gene expression, and synaptic signaling, among others. In this case, a leucine-rich repeat (LRR) protein, SHOC2 (Sur-8 homolog on chromosome 2), acts as both a regulatory subunit and a scaffold to the SHOC2-PP1C-RAS complex2. PP1C dephosphorylates specific residues on the RAS protein, leading to its activation.
SHOC2 contains 20 LRR repeats of 22-24 amino acids each, and folds into a solenoid structure. The transition of the unbound form of SHOC2 to its bound form in the SHOC2-PP1C-RAS complex is predominantly determined by the first ANM mode. The intrinsic dynamics of SHOC2 also plays a significant role in facilitating the assembly of the ternary complex with PP1C and RAS.
Scaffolding proteins’ intrinsic dynamics facilitate the assembly of phosphatases. We illustrate how the intrinsic dynamics of PR65 (subunit A) favors its conformational change upon complexation with the catalytic unit (the dimer AC) and that of AC favors formation of the trimer ABC (of PP2A), or the complex formed between PP2A and the 12-meric integrator protein. PP2A plays diverse regulatory roles in cell signaling and cellular processes.3
References
1Cohen, P. T., Protein phosphatase 1--targeted in many directions. J Cell Sci 2002, 115 (Pt 2), 241-56.
211. Liau, N. P. D.; Johnson, M. C.; Izadi, S.; Gerosa, L.; Hammel, M.; Bruning, J. M.; Wendorff, T. J.; Phung, W.; Hymowitz, S. G.; Sudhamsu, J., Structural basis for SHOC2 modulation of RAS signalling. Nature 2022, 609 (7926), 400-407.
4Kaynak BT, Dahmani ZL, Doruker P, Banerjee A, Yang SH, Gordon R, Itzhaki LS, Bahar I (2023) Cooperative mechanics of PR65 scaffold underlies the allosteric regulation of the phosphatase PP2A Structure 31, 607-618.