Chaperones and Co-Chaperones
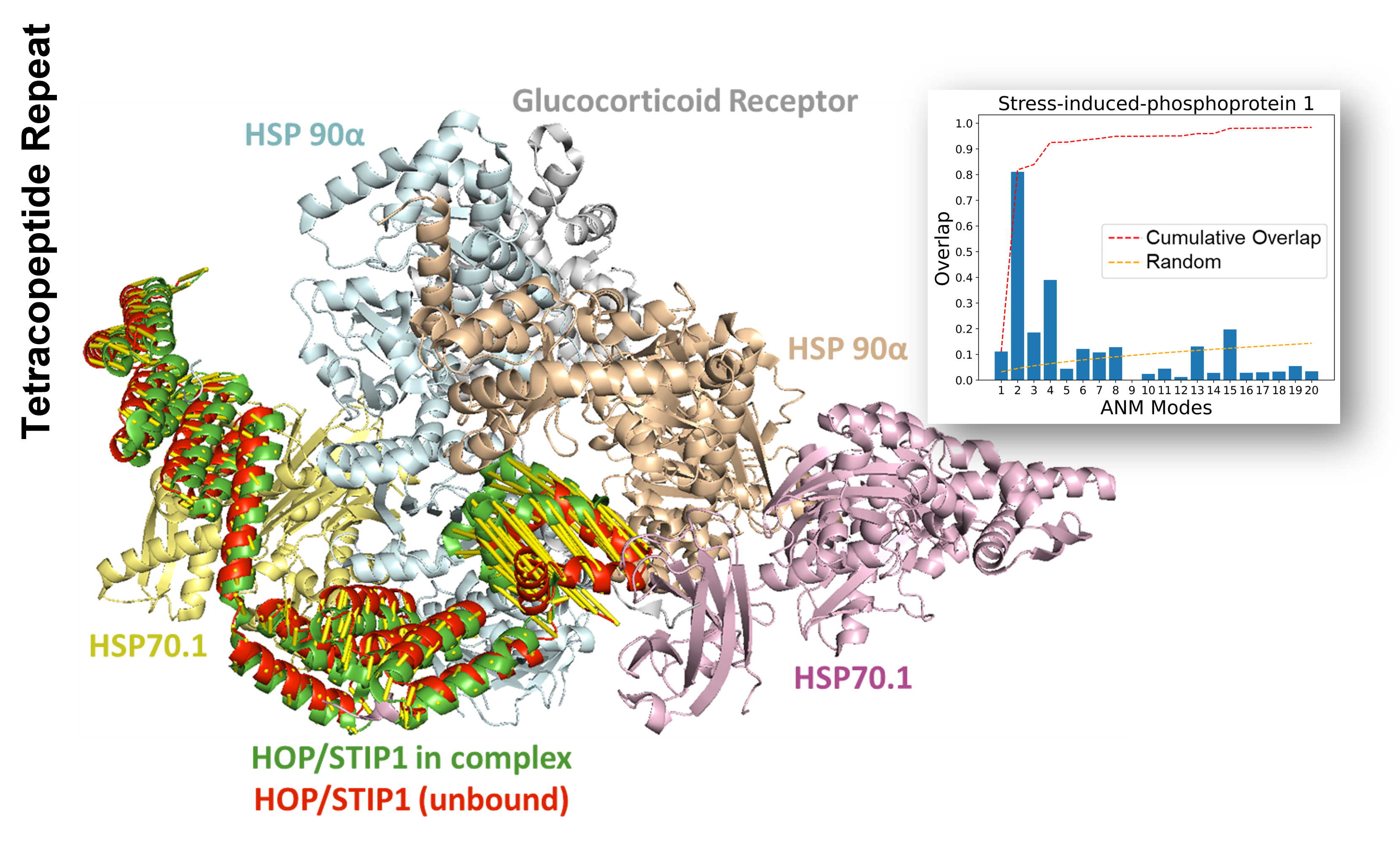
Chaperones and co-chaperones interact with client proteins, aiding in their folding or unfolding and refolding. The inherent dynamics of chaperones and co-chaperones make them predisposed to bind to their substrates. For instance, stress-induced-phosphoprotein 1 (STIP1)1 is a co-chaperone that regulates ternary complexes involving Hsp90 and Hsp70. It stabilizes the complex through tetratricopeptide repeats and assists in reorienting substrates before transferring them to Hsp90.
Another example is the ER membrane protein complex subunit 2 (EMC2)2, which stabilizes the EMC complex and aids in protein movement into the ER membrane. Hsp70-binding protein 1 (HSPBP1) acts as a co-chaperone for Hsp90 and Hsp70, promoting protein degradation by inhibiting the chaperonin's ATPase activity. It utilizes its flexible N-terminal extension to facilitate Hsp70 substrate removal.
Peptidyl-prolyl cis-trans isomerase, also known as FKBP53, is a seven TPR protein that regulates steroid hormone receptors in complex with HSP90. The unbound and bound conformations of FKBP5 in the Hsp90:FKBP51:p23 complex show significant conformational changes.
Overall, the first few modes of global motion capture the conformational changes of these co-chaperones, providing insights into their dynamic interactions with client proteins.
References
1da Fonseca, A. C. C.; Matias, D.; Geraldo, L. H. M.; Leser, F. S.; Pagnoncelli, I.; Garcia, C.; do Amaral, R. F.; da Rosa, B. G.; Grimaldi, I.; de Camargo Magalhães, E. S.; Cóppola-Segovia, V.; de Azevedo, E. M.; Zanata, S. M.; Lima, F. R. S., The multiple functions of the co-chaperone stress inducible protein 1. Cytokine Growth Factor Rev 2021, 57, 73-84.
2Hegde, R. S., The Function, Structure, and Origins of the ER Membrane Protein Complex. Annu Rev Biochem 2022, 91, 651-678.
3Soto, O. B.; Ramirez, C. S.; Koyani, R.; Rodriguez-Palomares, I. A.; Dirmeyer, J. R.; Grajeda, B.; Roy, S.; Cox, M. B., Structure and function of the TPR-domain immunophilins FKBP51 and FKBP52 in normal physiology and disease. J Cell Biochem 2023.