Ensemble analysis: dynamics from PCA of multiple structures.¶
This example shows how to run an ensemble analysis workflow in Scipion by importing several structures and building an ensemble, and analysing it via PCA. We will use the SARS-CoV-2 spike as in [KJ23].
See [KJ23] for more information about the Scipion-EM-ProDy plugin and more examples.
Getting started¶
You can open the Scipion Projects window from the command line as follows:
scipion3
From here, create a new project and call it prody_tutorial_ensemble. Then, select the ProDy protocols tree from the dropdown on the left.
Advanced case: many heterogeneous structures¶
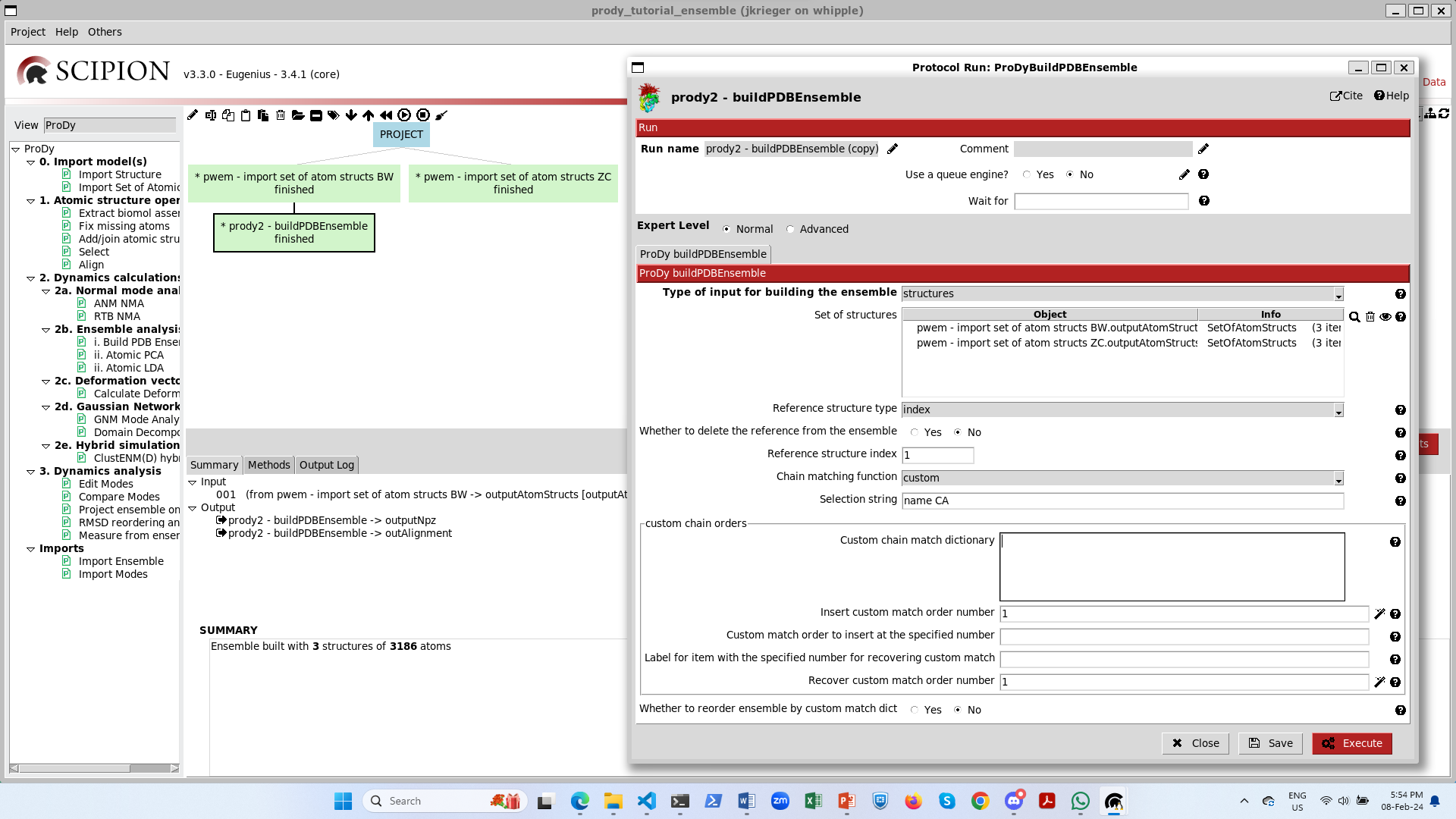
If we import structures from another paper that uses a different nomenclature for chains, then it becomes more difficult to align them. One good solution for this is to provide a custom chain matching dictionary as follows.
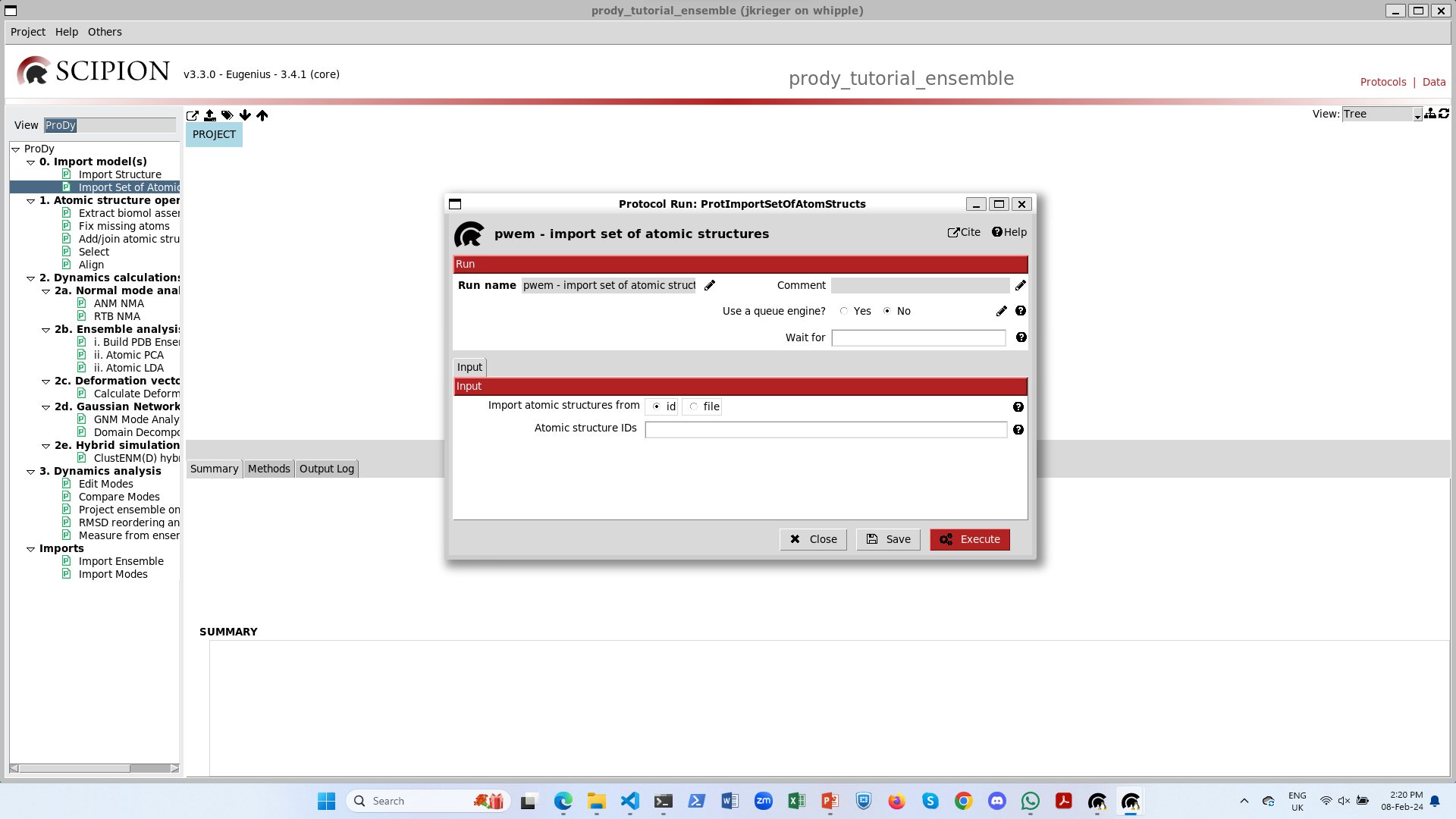
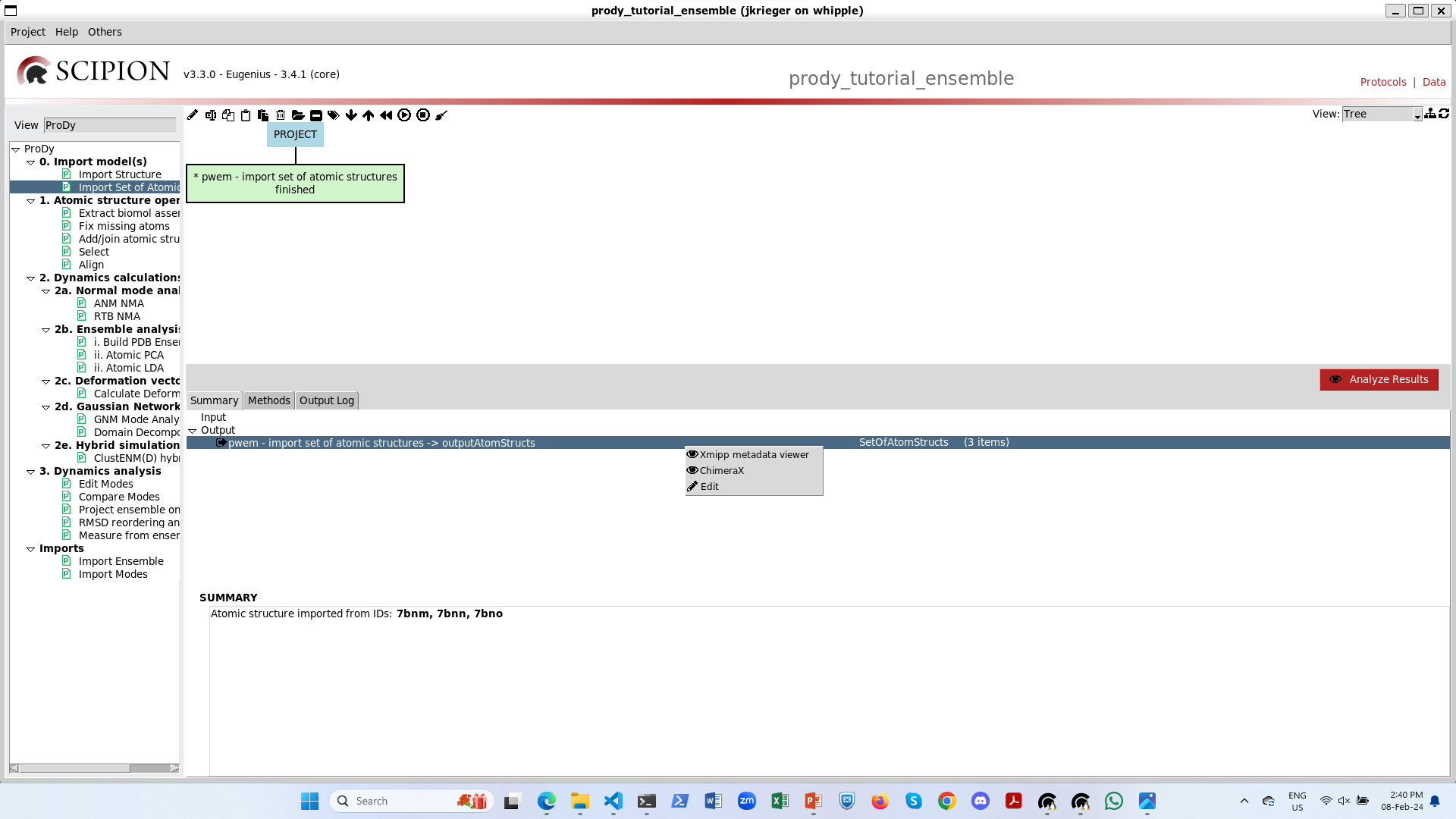
Let’s rename and duplicate the import protocol and import the structures from Zhang and Cai et al. 2021 ([ZC21]): 7krq, 7krr, 7krs.
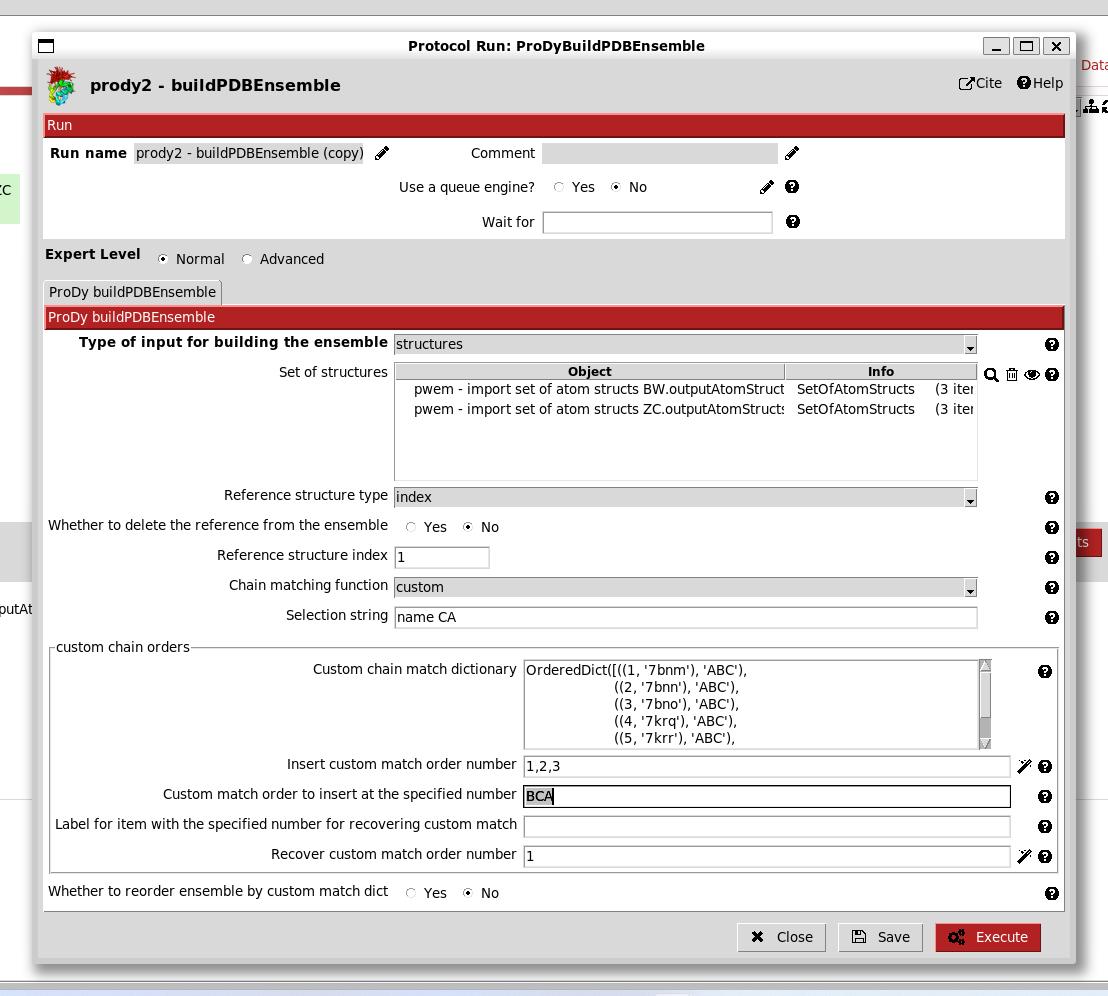
When we select the custom match option on the form, then we get an extra section with a wizard for creating a matching dictionary:
Initially, there is a big empty box, but when we click the magic wand underneath it, then it populates with the chain orders in each of the structures.
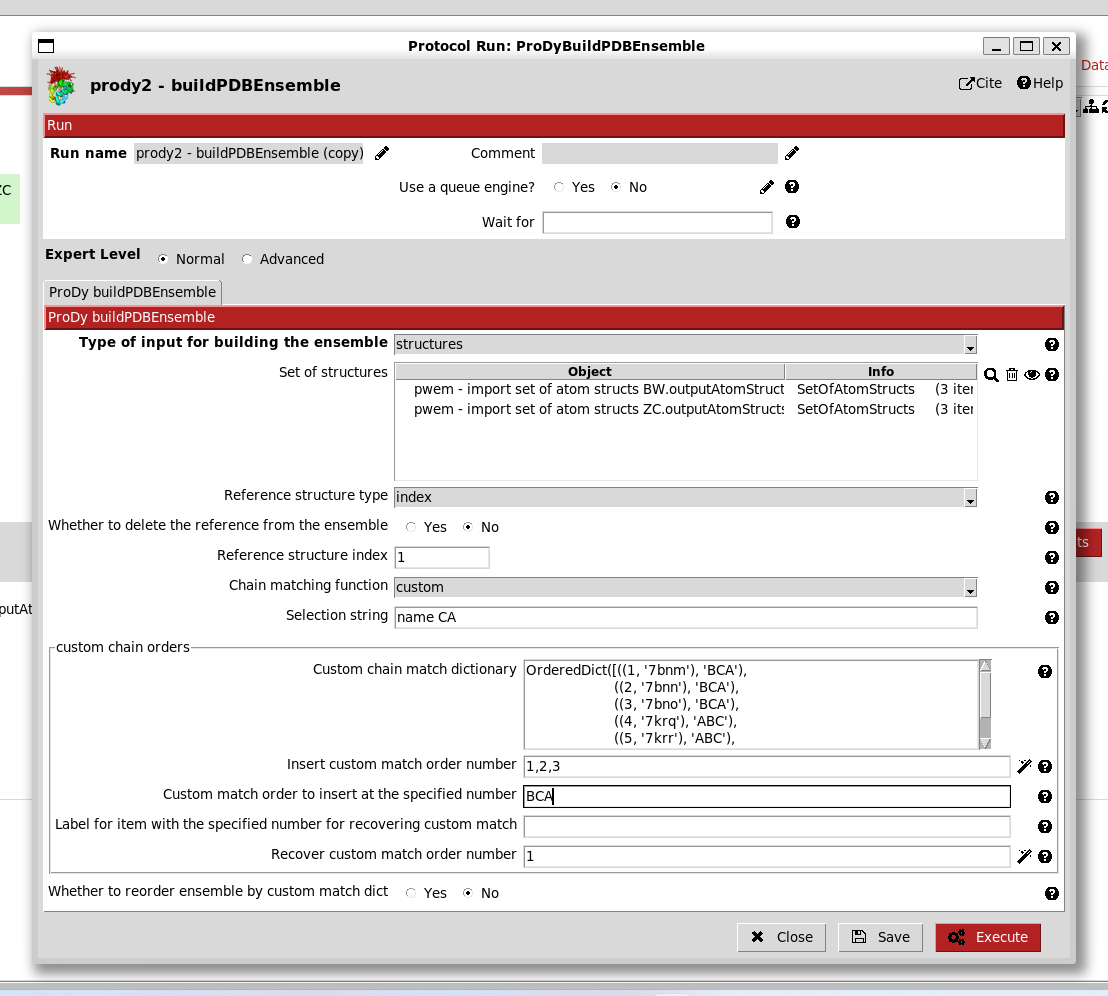
Following our previous studies ([KJ23] and [GT22]), we know that chain order BCA in the first structures matches chain order ABC in the second set. Therefore, we can change the orders at position numbers 1,2,3 to BCA as follows:
When we then click the magic wand again, the match dictionary changes accordingly:
This applies when we use the unite_chains advanced option, so we set this to yes.
We can now execute the protocol and get the correct ensemble. The box gets the same name as the other one with “(copy)” added on the end, which is good enough for now.
When we click the red analyse button, we now see that all 1-up structures have the same RBD up and that RBD is also up in the 2-up structure.
Step 2-ii: PCA¶
Now we are ready to analyse the ensembles by PCA, which is step 2-ii. We can do this with the default options for each ensemble, copying the protocol again for the second one.
By default, it uses half the threads, so we can run both at the same time.
Scipion also has random failures sometimes, so if this happens, just right-click the protocol box and select restart.
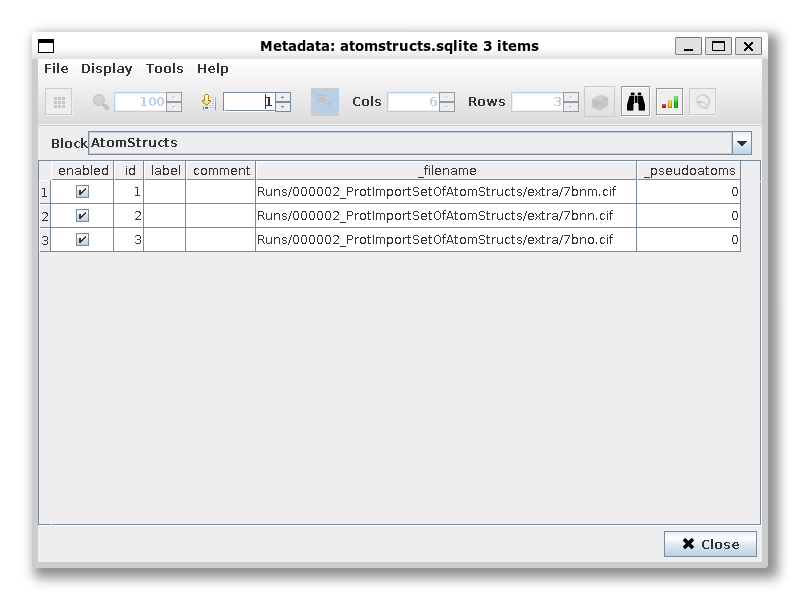
The protocol and output SetOfPrincipalComponents, like the NMA protocol and SetOfNormalModes, can open the NMWiz viewer or with the metadata viewer. In this case, the metadata viewer can be helpful for looking at the fractional variance.
In the NMWiz viewer, we can see that the first mode features concerted opening and closing of two RBDs, while the second features anti-correlated opening of these two RBDs.
For the PCA from the larger ensemble, we also have additional meaningful modes of variation, particular PC3 which shows a locking and unlocking of the RBDs with respect to each other and the NTDs.
Step 3: analysis 1 - compare modes¶
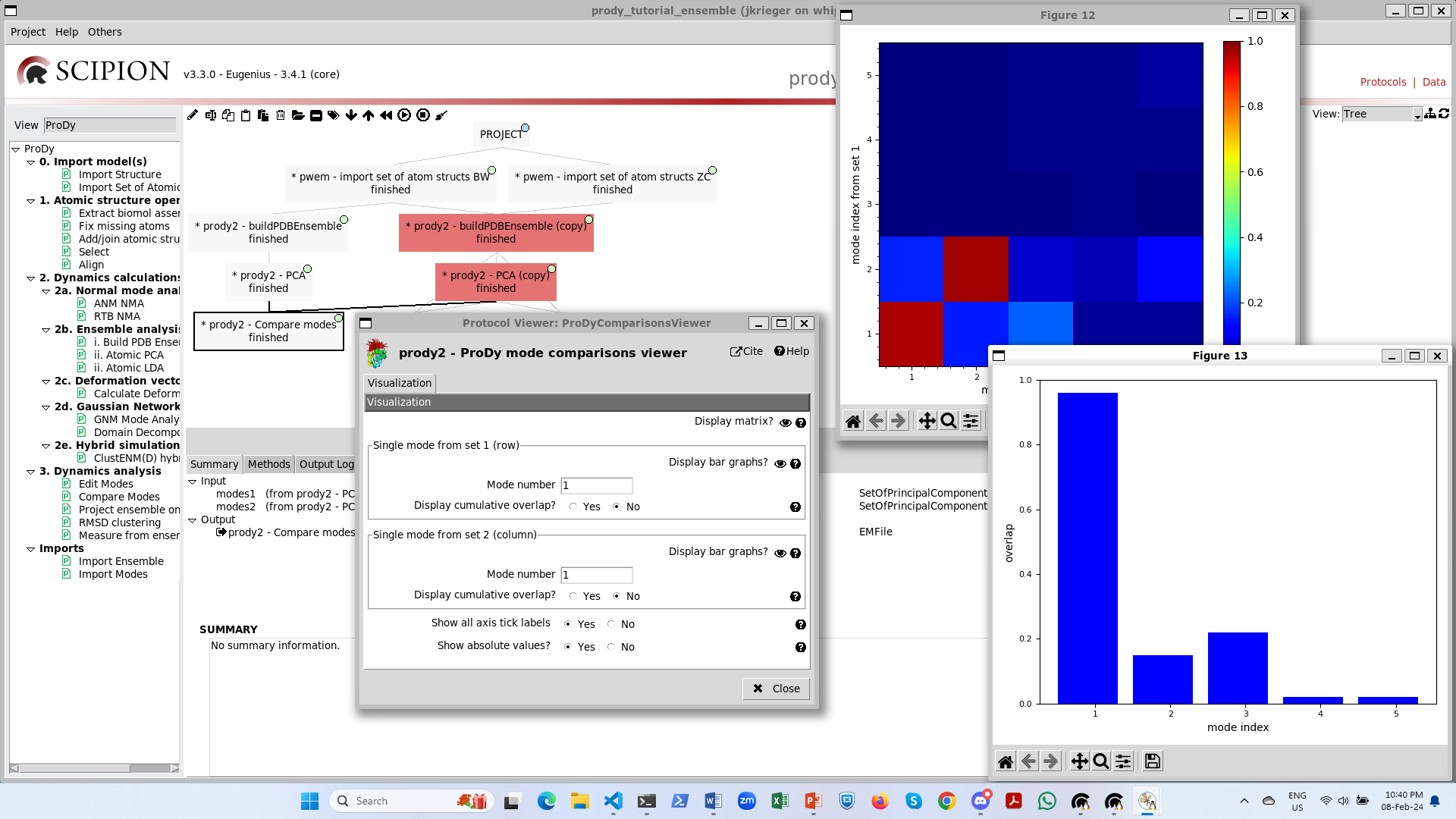
The comparison between two sets of modes is slightly different to the comparison of a single vector against a set of modes. In this case, we have an overlap matrix.
This gives us additional options of matching modes and slicing out the diagonal, but we will not pick these now.
In the end, the viewer gives us ways of interacting with this matrix. Either, we can plot the colour map like
showOverlapTable() or we can select rows to plot like showOverlap() as in the single deformation
vector case. We then get the following outputs:
Step 3: analysis 2 - project ensembles on PCA landscapes¶
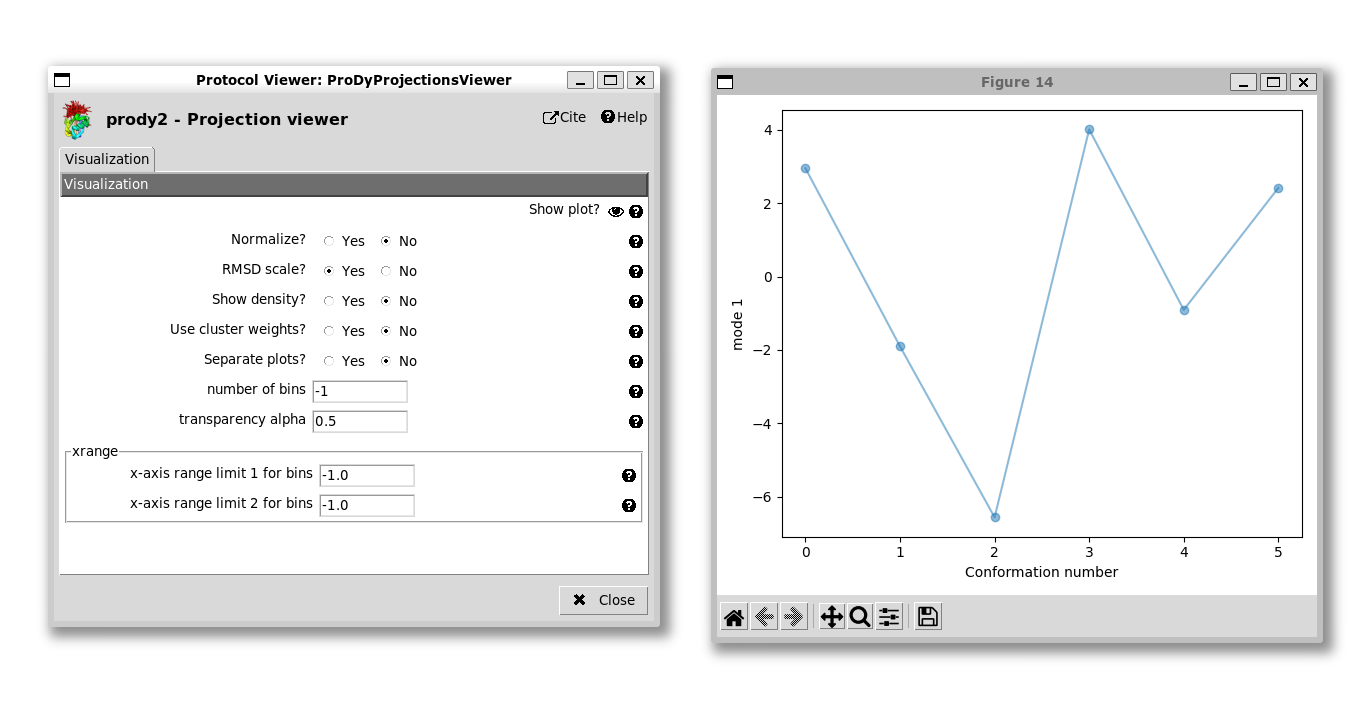
Finally, we can run the projection protocol to project the members of the ensemble onto the PCA components, allowing us to visualise the conformational landscapes. We have options of projecting onto 1, 2 or 3 modes.
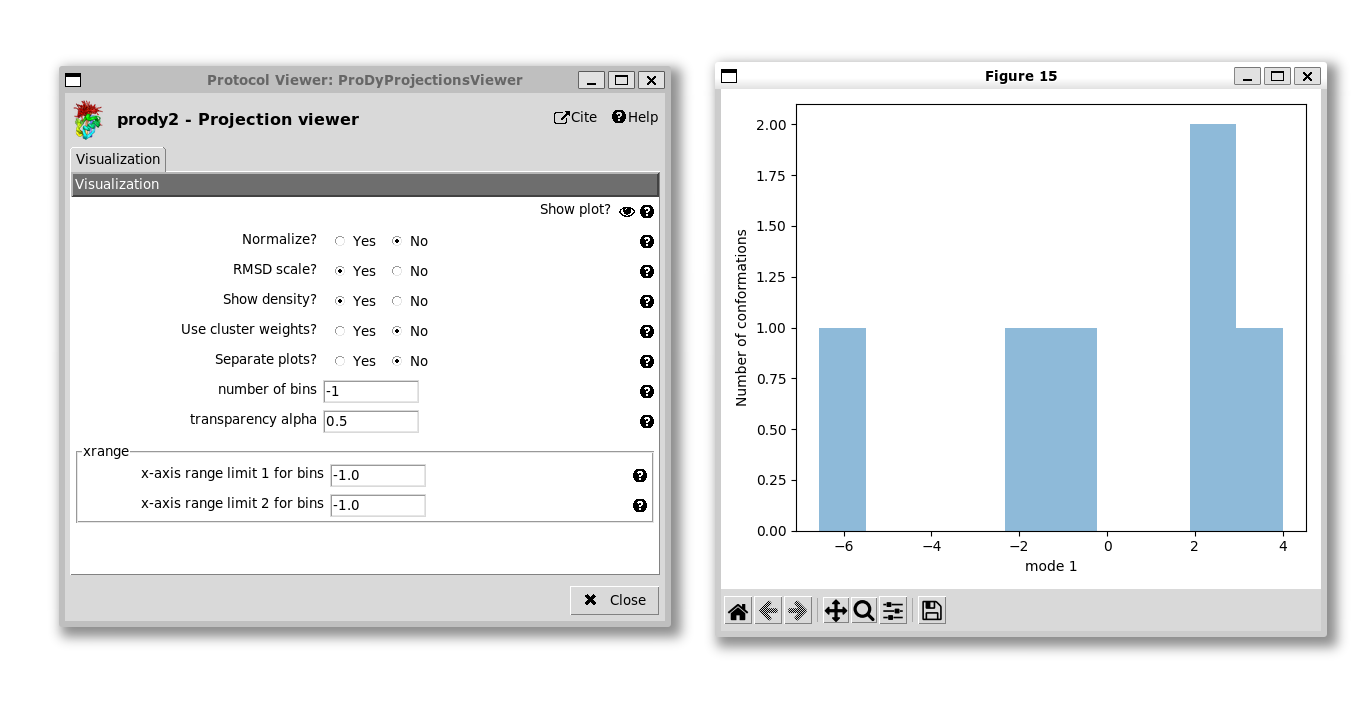
If we project onto 1 mode, then the default option is to get a line graph with points for each structure with the conformation number on the x-axis and the PC coordinate along the y-axis:
We can change this to a histogram by setting “Show density” to yes:
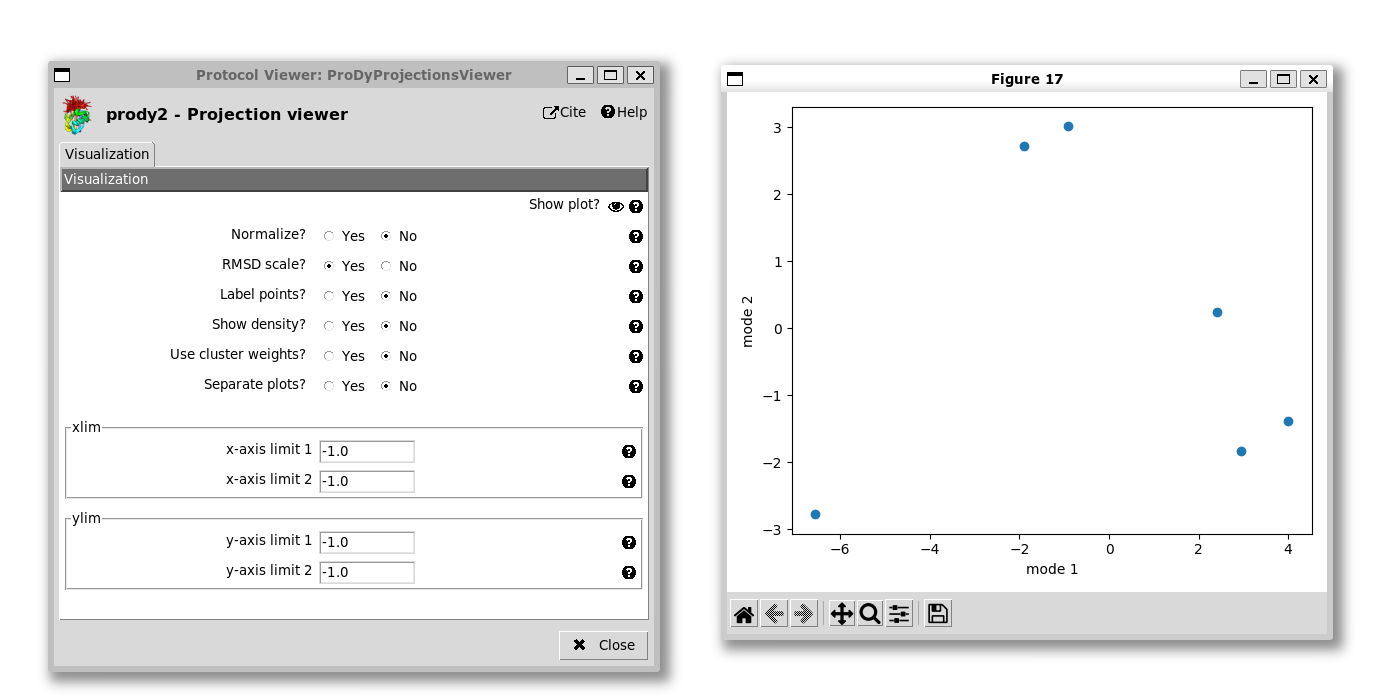
For the 2D case, the default plot is a scatter graph with each point being a structure and the axes being the two selected PCs:
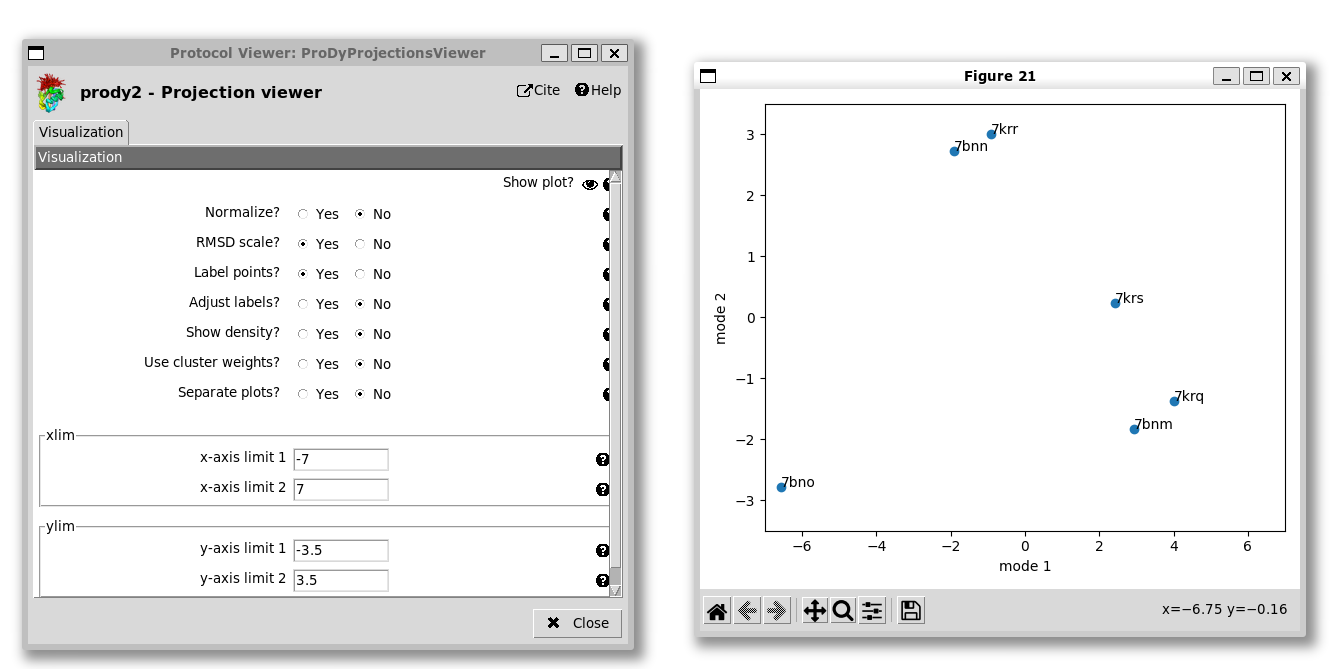
Unlike the 1D case, it’s not easy to know which point is which, so we have an option to label the points. As in the ProDy API, we can adjust the labels if they are overlapping, but that’s not necessary here. We also have the option of setting the axis ranges, which is useful for getting a more equal view, e.g. setting the x-axis from -7 to +7 and the y-axis from -3.5 to +3.5 as we do here:
We see that the 2-up structure (PDB: 7bno) locates to the bottom left corner and the 3-down structures (PDB: 7bnm and 7krq) are near the the bottom right corner, separated mostly by PC1.
The 1-up structures (PDB: 7bnn and 7krr) lie near the middle of the top, being separated from the 3-down structures by a combination of PCs 1 and 2. The 1-up, 1-intermediate structure (PDB: 7krs) lies along the line between the two end states as expected.
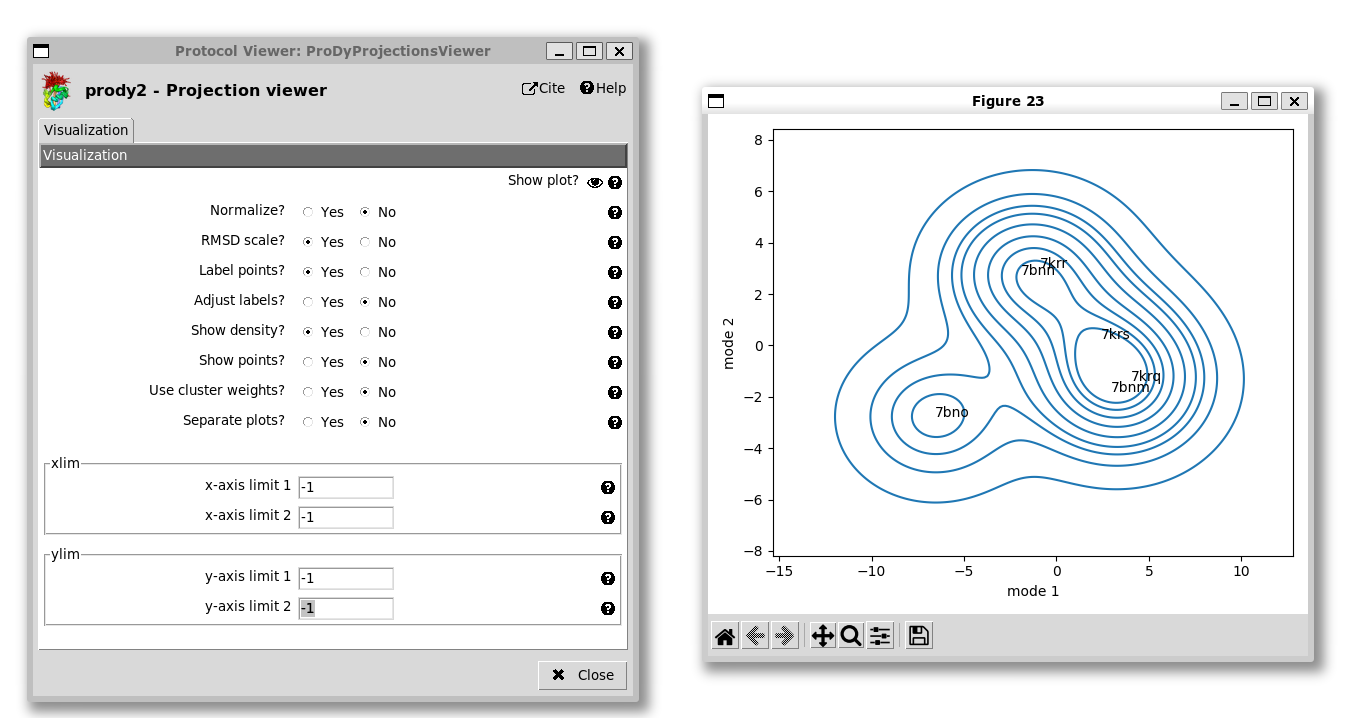
We also have the option of showing a kernel density estimate of the landscape, which requires a bigger plot space, so we set the x and y limits back to -1 to allow them to take default values:
The 3D projection works in a similar way to the 2D with the absence of the density representation. It gives a 3D scatter plot that can be rotated as in the ProDy API (not shown here), which shows that PC3 mostly separates the structures from the two papers (excluding the 2-up), which may be due to differences in the constructs such as stabilising proline mutations in [BW21] and absent in [ZC21].
There are also other options for calculating distances, angles and dihedrals from these ensembles and clustering them by RMSD.
| [BW21] | (1, 2) Benton DJ, Wrobel AG, Roustan C, Borg A, Xu P, Martin SR, Rosenthal PB, Skehel JJ, Gamblin SJ. The effect of the D614G substitution on the structure of the spike glycoprotein of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2021 118(9): e2022586118. |
| [ZC21] | (1, 2) Zhang J, Cai Y, Xiao T, Lu J, Peng H, Sterling SM, Walsh RM Jr, Rits-Volloch S, Zhu H, Woosley AN, Yang W, Sliz P, Chen B. Structural impact on SARS-CoV-2 spike protein by D614G substitution. Science 2021 372(6541): 525-530. |
| [GT22] | Ginex T, Marco-Marín C, Wieczór M, Mata CP, Krieger J, Ruiz-Rodriguez P, López-Redondo ML, Francés-Gómez C, Melero R, Sánchez-Sorzano CÓ, Martínez M, Gougeard N, Forcada-Nadal A, Zamora-Caballero S, Gozalbo-Rovira R, Sanz-Frasquet C, Arranz R, Bravo J, Rubio V, Marina A; IBV-Covid19-Pipeline; Geller R, Comas I, Gil C, Coscolla M, Orozco M, Llácer JL, Carazo JM. The structural role of SARS-CoV-2 genetic background in the emergence and success of spike mutations: The case of the spike A222V mutation. PLoS Pathog. 2022 18(7): e1010631. |