Advances in structure-function analyses and computational biology have enabled a deeper understanding of how excitatory amino acid transporters (EAATs) mediate chloride permeation and substrate transport. However, the mechanism of structural coupling between these functions remains to be established. Using a combination of molecular modeling, substituted cysteine accessibility, electrophysiology and glutamate uptake assays, we identified a chloride-channeling conformer, iChS, transiently accessible as EAAT1 reconfigures from substrate/ion-loaded into a substrate-releasing conformer. Opening of the anion permeation path in this iChS is controlled by the elevator-like movement of the substrate-binding core, along with its wall that simultaneously lines the anion permeation path (global); and repacking of a cluster of hydrophobic residues near the extracellular vestibule (local). Moreover, our results demonstrate that stabilization of iChS by chemical modifications favors anion channeling at the expense of substrate transport, suggesting a mutually exclusive regulation mediated by the movement of the flexible wall lining the two regions.
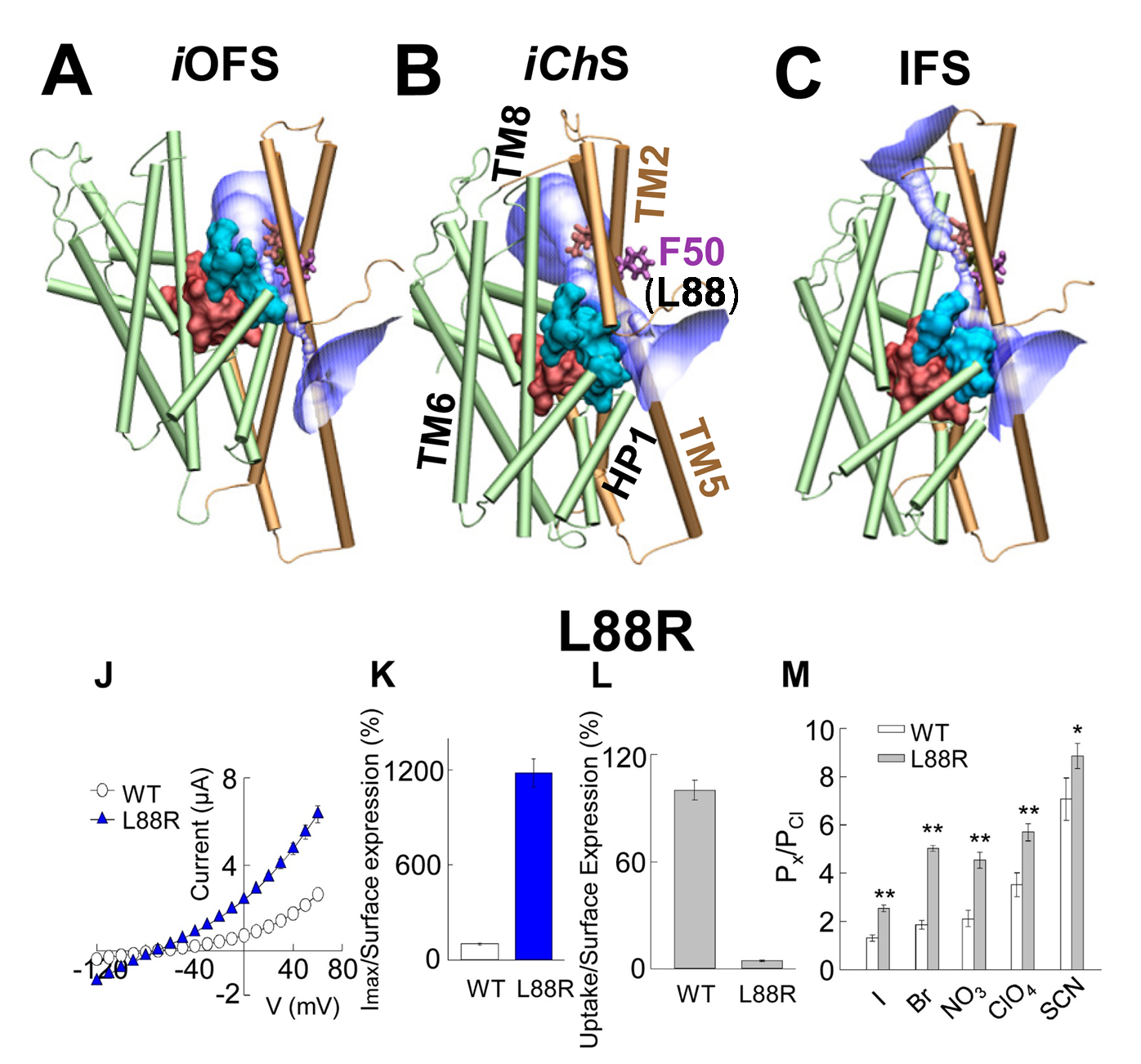
Combination of computational and experimental studies of anion conductance mechanism of excitatory amino acid (EAAT) transporters. (Upper panel) Simultaneous Cl channeling /Glu- transport involves a moving wall (cyan) which separates the substrate-binding (pink) and anion permeation regions (purple). The structures are shown for (A) intermediate outward-facing open state (iOFS; PDB: 3V8G); (B) intermediate channeling state (iChS) identified by our computational study; and (C) inward-facing state (IFS; PDB: 3KBC) in GltPh. F50 (homologous L88 in EAAT1) in Gltph serves as a gate. (lower panel) (J) Current-voltage relationship measured in oocytes expressing WT EAAT1 or L88R. (K) The maximum current amplitude at +60 mV from panel J was normalized by surface expression to reflect the actual difference in current amplitude. (L) The same surface expression was used to normalize radiolabeled glutamate uptake measured in oocytes expressing WT EAAT1 or L88R. More details see Cheng et al eLife 2017
|

